Vaccines
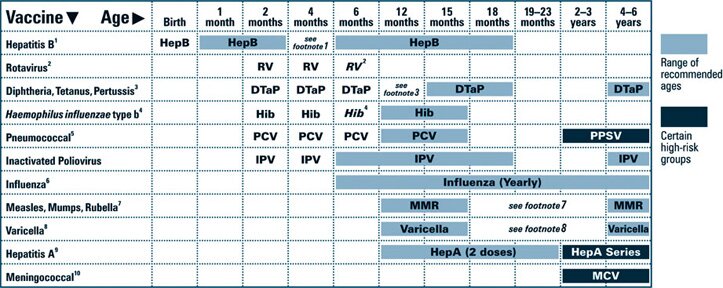
This schedule indicates the recommended ages for routine administration of currently licensed vaccines, as of December 1, 2008, for children aged 0 through 6 years. Any dose not administered at the recommended age should be administered at a subsequent visit, when indicated and feasible. Licensed combination vaccines may be used whenever any component of the combination is indicated and other components are not contraindicated and if approved by the Food and Drug Administration for that dose of the series. Providers should consult the relevant Advisory Committee on Immunization Practices statement for detailed recommendations, including high-risk conditions: http://www.cdc.gov/vaccines/pubs/acip-list.htm. Clinically significant adverse events that follow immunization should be reported to the Vaccine Adverse Event Reporting System (VAERS). Guidance about how to obtain and complete a VAERS form is available at http://www.vaers.hhs.gov or by telephone, 800-822-7967.
1. Hepatitis B vaccine (HepB). (Minimum age: birth)
At birth:
- Administer monovalent HepB to all newborns before hospital discharge.
- If mother is hepatitis B surface antigen (HBsAg)-positive, administer HepB and 0.5 mL of hepatitis B immune globulin (HBIG) within 12 hours of birth.
- If mother’s HBsAg status is unknown, administer HepB within 12 hours of birth. Determine mother’s HBsAg status as soon as possible and, if HBsAg-positive, administer HBIG (no later than age 1 week).
After the birth dose:
- The HepB series should be completed with either monovalent HepB or a combination vaccine containing HepB. The second dose should be administered at age 1 or 2 months. The final dose should be administered no earlier than age 24 weeks.
- Infants born to HBsAg-positive mothers should be tested for HBsAg and antibody to HBsAg (anti-HBs) after completion of at least 3 doses of the HepB series, at age 9 through 18 months (generally at the next well-child visit).
-
4-month dose:
- Administration of 4 doses of HepB to infants is permissible when combination vaccines containing HepB are administered after the birth dose.
2. Rotavirus vaccine (RV). (Minimum age: 6 weeks)
- Administer the first dose at age 6 through 14 weeks (maximum age: 14 weeks 6 days). Vaccination should not be initiated for infants aged 15 weeks or older (i.e., 15 weeks 0 days or older).
- Administer the final dose in the series by age 8 months 0 days.
- If Rotarix is administered at ages 2 and 4 months, a dose at 6 months is not indicated.
3. Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP). (Minimum age: 6 weeks)
- The fourth dose may be administered as early as age 12 months, provided at least 6 months have elapsed since the third dose.
- Administer the final dose in the series at age 4 through 6 years.
4. Haemophilus influenzae type b conjugate vaccine (Hib). (Minimum age: 6 weeks)
- If PRP-OMP (PedvaxHIB® or Comvax® [HepB-Hib]) is administered at ages 2 and 4 months, a dose at age 6 months is not indicated.
- TriHiBit® (DTaP/Hib) should not be used for doses at ages 2, 4, or 6 months but can be used as the final dose in children aged 12 months or older.
5. Pneumococcal vaccine. (Minimum age: 6 weeks for pneumococcal conjugate vaccine [PCV]; 2 years for pneumococcal polysaccharide vaccine [PPSV])
- PCV is recommended for all children aged younger than 5 years. Administer 1 dose of PCV to all healthy children aged 24 through 59 months who are not completely vaccinated for their age.
- Administer PPSV to children aged 2 years or older with certain underlying medical conditions (see MMWR 2000;49[No. RR-9]), including a cochlear implant.
6. Influenza vaccine. (Minimum age: 6 months for trivalent inactivated influenza vaccine [TIV]; 2 years for live, attenuated influenza vaccine [LAIV])
- Administer annually to children aged 6 months through 18 years.
- For healthy nonpregnant persons (i.e., those who do not have underlying medical conditions that predispose them to influenza complications) aged 2 through 49 years, either LAIV or TIV may be used.
- Children receiving TIV should receive 0.25 mL if aged 6 through 35 months or 0.5 mL if aged 3 years or older.
- Administer 2 doses (separated by at least 4 weeks) to children aged younger than 9 years who are receiving influenza vaccine for the first time or who were vaccinated for the first time during the previous influenza season but only received 1 dose.
7. Measles, mumps, and rubella vaccine (MMR). (Minimum age: 12 months)
- Administer the second dose at age 4 through 6 years. However, the second dose may be administered before age 4, provided at least 28 days have elapsed since the first dose.
8. Varicella vaccine. (Minimum age: 12 months)
- Administer the second dose at age 4 through 6 years. However, the second dose may be administered before age 4, provided at least 3 months have elapsed since the first dose.
- For children aged 12 months through 12 years the minimum interval between doses is 3 months. However, if the second dose was administered at least 28 days after the first dose, it can be accepted as valid.
9. Hepatitis A vaccine (HepA). (Minimum age: 12 months)
- Administer to all children aged 1 year (i.e., aged 12 through 23 months). Administer 2 doses at least 6 months apart.
- Children not fully vaccinated by age 2 years can be vaccinated at subsequent visits.
- HepA also is recommended for children older than 1 year who live in areas where vaccination programs target older children or who are at increased risk of infection. See MMWR 2006;55(No. RR-7).
10. Meningococcal vaccine. (Minimum age: 2 years for meningococcal conjugate vaccine [MCV] and for meningococcal polysaccharide vaccine [MPSV])
- Administer MCV to children aged 2 through 10 years with terminal complement component deficiency, anatomic or functional asplenia, and certain other high-risk groups. See MMWR 2005;54(No. RR-7).
- Persons who received MPSV 3 or more years previously and who remain at increased risk for meningococcal disease should be revaccinated with MCV.